MgCl2 is an ionic compound because chemical bonds in the molecule are formed by the transfer of electrons among Mg and Cl atoms. It behaves as a typical ionic halide and is solid at room.

Steps To Naming Ionic And Covalent Compounds Chemistry Chemistry Class Names
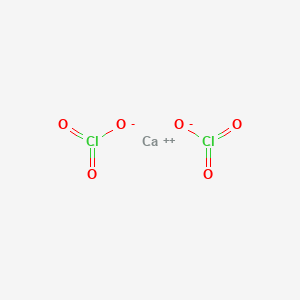
Calcium chlorate CaClO32 or CaCl2O6 CID 24978 - structure chemical names physical and chemical properties classification patents literature biological.

. Yes baking soda is an ionic compound. MgCl2 is an Ionic compound not a covalent. Classify the following compounds as having covalent or ionic bonds.
Why is Calcium Chloride covalent. Calcium chlorate is produced by passing chlorine gas through a hot suspension of calcium hydroxide in water producing calcium hypochlorite which disproportionates when heated with. Anhydrous calcium chloride crystallizes in the orthorhombic.
Ad Market Leader in Innovative Organic Inorganic Chemicals. Yescalcium chloride is ionic. Yescalcium chloride is ionicCaCl2 is a salt of calcium and chlorine.
Since the gap of electronegativity value between magnesium 131 and chlorine 316 is large therefore the bond formed in Magnesium. Covalent bonds only happen between two non-metals. Does calcium dichloride have ionic or covalent bonds.
Chemical bond A chemical bond is a lasting attraction between atoms ions or. It contains a metal- calcium. Ionic AND Covalent Compounds.
See the sites suggested by Lance then write in the electronegativity values and post them give the difference and suggest ionic or covalent. No but these answers are closer than the first set. Calcium chloride is not a covalent bond.
They all have an outer shell with two electrons and are very reactive. Hence calcium chloride is an ionic compound. View Available Hint s Reset Help carbon dioxide.
To tell if CaCl2 Calcium chloride is ionic or covalent also called molecular we look at the Periodic Table that and see that Ca is a metal and Cl is a no. Calcium chloride CaCl2 is ionic bond What is chemical bond ionic bond covalent bond. Yes calcium is defined as a metal because of both its physical and chemical traits.
Formulas vs Names and Covalent Compounds. So is MgCl2 ionic or covalent. Ad Market Leader in Innovative Organic Inorganic Chemicals.
Drag the appropriate compounds to their respective bins. Calcium has both ionic and covalent bonds. This is a combination of sets Ionic Compounds.
It behaves as a typical ionic halide and is solid at room temperatureCalcium is a metal which is bonded to non-metal. NaCl Sodium chloride AlBr 3 Aluminum bromide Ca 3P 2 Calcium phosphide SrI 2 Strontium iodide FeCl 2 IronII chloride or ferrous chloride 2. Calcium chloride is highly soluble in water owing to its ionic nature.
The only difference is the size of cations Cs being larger than Na and hence Na will have more polarising power than Cs and hence NaCl will be more covalent than CsCl or in. Formulas vs Names STUDY. Baking soda is composed of sodium ions Na and bicarbonate ions HCO3 also called hydrogen carbonate ions in a 11 ratio.
CaCl2 is a salt of calcium and chlorine. Is calcium chloride an ionic or covalent bond. Ionic Compounds Containing a Metal and a.
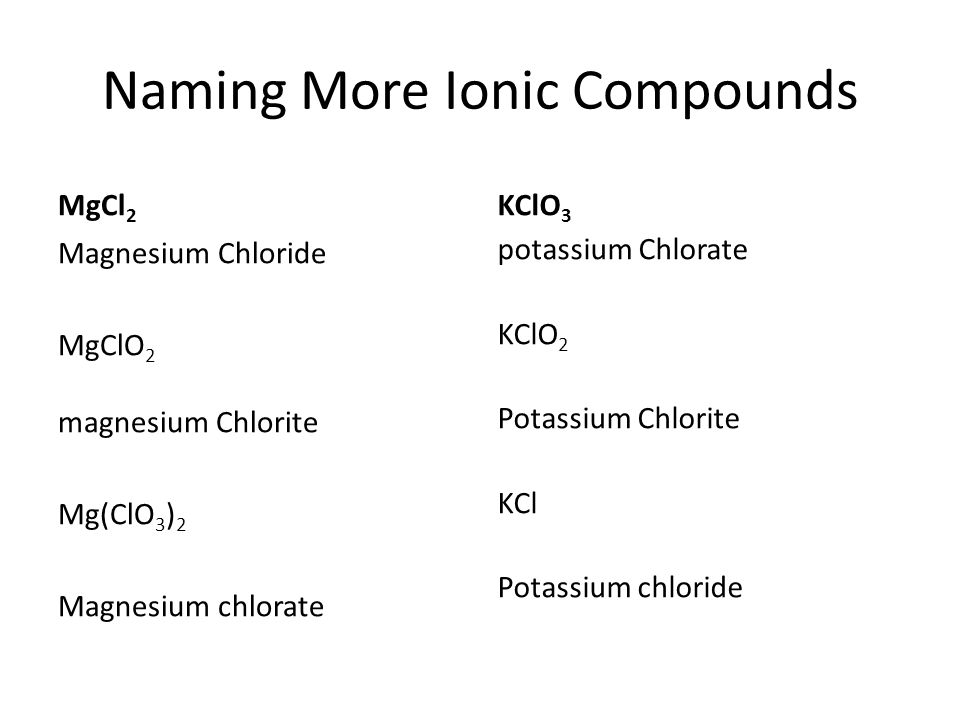
Naming Compounds Writing Formulas Ppt Video Online Download

Solubility Rules Solubility Of Common Ionic Compounds Sigma Aldrich Ionic Compound Solubility Chemical Chart
0 Comments